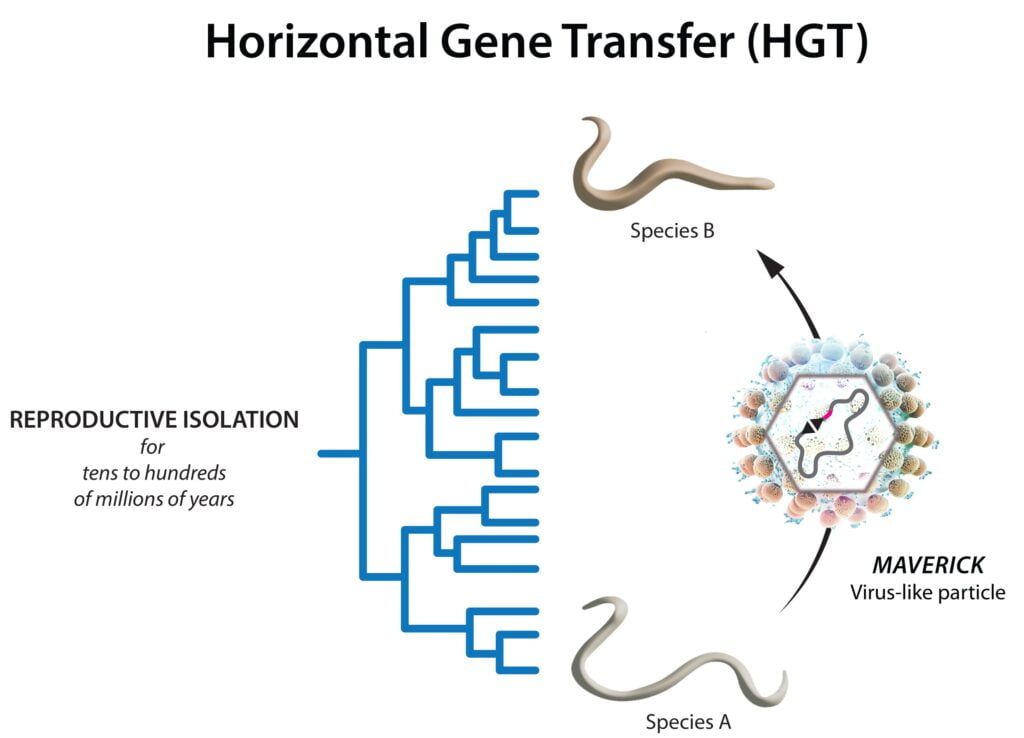
Scientists have known for years that genes can be transferred from one species to another, both in animals and plants. However, the mechanism by which such unexpected events occur is not known. Now, researchers from the laboratory of Alejandro Burga at the Institute of Molecular Biotechnology (IMBA) of the Austrian Academy of Sciences have identified a horizontal gene transfer (HGT) vector in worms. The results, published in Sciencemay lead to the release of other HGT vectors in eukaryotes and may find applications in insect control.
Fish that live in the Arctic and Antarctic seas have evolved clever ways to protect their blood and muscles from freezing in temporary waters. One of the mechanisms of such adaptation is the modification of genes that produce antifreeze proteins. However, 10 years ago, scientists were shocked to discover that two species of herring and smelts—two completely different species—have the exact same antifreeze protein in their bodies, indicating gene transfer between them.
Examples like these raise the question: How can genes “jump” between very different species? This rare phenomenon, known as HGT, has long puzzled ecologists. And although HGT innovations have been discovered in all areas of life over the years, the mechanisms behind these transfers are still largely unknown.
Now, scientists from Alejandro Burga’s team at IMBA not only have an HGT event in animals, but also realize one of the things they have been looking for for a long time. Using genetic engineering, Burga and his team demonstrated an HGT event between two species of worms that are as different as humans and fish. More importantly, they could identify the cause: a family of transposon-like viruses called Mavericks.
Pinning the culprit: Mavericks as HGT vectors
“Mavericks were previously known as a group of transposons, but our work links them to HGT for the first time,” says IMBA team leader Alejandro Burga, co-author of the study. “We knew that HGT happened among animal species, but we didn’t know how. This is the first time we’ve nailed down a culprit,” adds co-first author Sonya Widen, a postdoctoral fellow in the Burga lab.
When Mavericks were discovered in the mid-2000s, they were initially thought to be giant transposons, self-inflicted genes that jump and propagate themselves into the genome to the detriment of their hosts. Mavericks have been rapidly reported in many branches of eukaryotes, including humans, confirming their ancient origins.
Transposons and viruses, nature’s melting pot?
Soon, evidence that Mavericks contained genes for virus storage, such as capsid and DNA polymerase, began to emerge. “The evolution of transposons and viruses is tightly coupled,” says Burga. However, the capsid and DNA polymerase are not sufficient to allow the transposon to escape from the genome of its host and infect cells of a very different group.
Now, IMBA researchers have found the missing link: Mavericks in the genomes of worms have found a protein called fusogen, a transmembrane protein that mediates membrane fusion between different cells. By finding fusogen, the authors hypothesize that the Mavericks worm was able to create small particles that can attach to the cell membrane of another organism and infect them.
“To our knowledge, no fusogen has been reported before in Mavericks. Therefore, we think that the Mavericks worm may take its sequence from a virus,” says Widen. “Transposons and viruses can be thought of as a melting pot of nature. Their interaction can have unexpected consequences and cause genomic changes,” says Burga.
Demonstrating the importance of HGT in larvae
In this study, the IMBA team led by Alejandro Burga and co-first authors Sonya Widen and Israel Campo Bes, a former master’s student in Burga’s lab, discovered HGT “by chance,” as Widen says. Instead, the team is studying the evolutionary origins of the selfish factor in the nematode Caenorhabditis briggsae. In their research, they were able to trace this selfish gene back to another nematode, C. plicata, which had a very similar copy.
This is surprising because C. briggsae and C. plicata are the only two reproductive species. “Their genomes are as different as those of humans and fish, yet they both have a common gene that shows recent HGT events,” says Campo Bes.
“By looking closely at the genetics of C. plicata, we found that the parental sequence that gave rise to the selfish gene in C. briggsae was placed within Maverick in C. plicata. “The selfish gene in C. briggsae shows how HGT affects the evolution of genomes,” Widen explains. The IMBA team went on to show that Mavericks are responsible for many independent HGT events among worms of different species and are found worldwide.
Importance of agriculture and medicine
IMBA scientists argue that the interaction between transposons and viruses is the most important factor in the representation of HGT. Although they still find it hard to believe their success, they realize the impact their findings can have on raising the bar for HGT. “I was sure that we were looking at the case of HGT when we saw these results in the lab, but I was sure that we would never know how it happened. However, the stars aligned,” says Burga, who is also. predict that Mavericks and similar virus-like organisms may represent HGT in vertebrates and other eukaryotes.
Finally, the team envisions potential applications in the laboratory and as strategies to control pathogens. “If Maverick-mediated HGT is shown to work in any nematode species, it could become a valuable tool. Beyond rigorous laboratory and research studies such as the modification of non-species nematode genes, such a tool may allow us, in the future, to change genetic nematode species that may be important in agriculture or medicine,” concludes Burga.
More information:
Sonya A. Widen et al, transposons as viruses cross the species barrier and drive the evolution of genetic disequilibrium, Science (2023). DOI: 10.1126/science.ade0705. www.science.org/doi/10.1126/science.ade0705
More news:
Science
Provided by IMBA- Institute of Molecular Biotechnology of the Austrian Academy of Sciences
#Transposons #viruses #cross #color #barrier #study #shows